Abstract
Background:
Fostamatinib is an oral small molecule spleen tyrosine kinase (Syk) inhibitor that was approved for treatment of adult patients with immune thrombocytopenia (ITP) in second-line therapy [1]. Syk inhibition prevents cytoskeletal rearrangements during phagocytosis allowing platelet survival in ITP. The efficacy of fostamatinib in adults with chronic ITP has been established in phase 3 clinical trials [2], and further studies have established fostamatinib as an effective second-line treatment [3]. However, fostamatinib treatment in elderly patients with ITP has not been well established. These individuals pose a particular challenge with a higher risk of bleeding [4], having multiple comorbidities, and may be at increased risk of toxicity from treatments such as fostamatinib.
Methods:
We performed a retrospective review of all elderly patients (age greater than or equal to 65 years) who had started on fostamatinib between June 1, 2021 and May 31, 2022 for the treatment of ITP at a single tertiary care centre to evaluate its efficacy and safety.
Results:
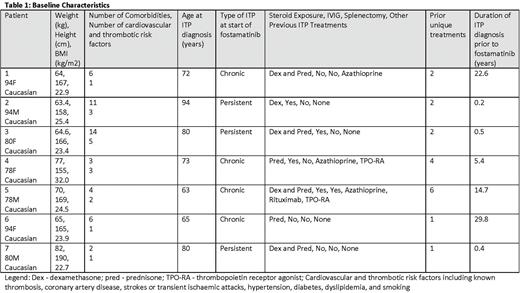
Seven patients, median age 80 years (range 78 - 94), four women and three men, all Caucasian background, with various comorbidities started fostamatinib 100mg oral twice daily as second or subsequent line therapy. Patients had a diagnosis of ITP for a median 6 years (range approximately 6 months - 30 years), had six comorbidities (range 2 - 14), and experienced 2 unique prior lines of ITP therapy (range 1 to 6).
Over 1290 days of fostamatinib exposure, two patients required dose escalation to 150mg oral twice daily while five patients remained on initial starting dose of 100mg twice daily in partial or complete response. The median platelet count at time of initiating fostamatinib was 25 x 109/L (range less than 10 - 193). Median time to response (defined any first platelet count greater than or equal to 30 x 109/L) was 19 days (range 0 - 181 days) with two patients responding rapidly (5 days and 19 days). Two patients required dose escalation, rescue therapy, and these same two patients discontinued fostamatinib after 175 days and 216 days of treatment. Treatment was tolerated in all patients with no thromboembolic events observed. One death was noted and unrelated to treatment. In keeping with the current recommendations, dose reduction was not required in any of the patients despite their advanced age.
Conclusions: Fostamatinib was effective and safe for the majority of our very elderly patients with ITP in second and subsequent line therapies over a cumulative 1290 days of exposure.
References: 1. Neunert, C. et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv 3, 3829-3866 (2019).
2. Bussel, J. et al. Fostamatinib for the treatment of adult persistent and chronic immune thrombocytopenia: Results of two phase 3, randomized, placebo-controlled trials. Am J Hematol 93, 921-930 (2018).
3. Boccia, R. et al. Fostamatinib is an effective second-line therapy in patients with immune thrombocytopenia. Br J Haematol 190, 933-938 (2020).
4. Cortelazzo, S. et al. High Risk of Severe Bleeding in Aged Patients With Chronic Idiopathic Thrombocytopenic Purpura. Blood 77, 31-33 (1991).
Disclosures
Hsia:Medison: Consultancy, Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.